|
 |
 |

|
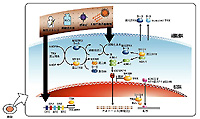
| Thioredoxin was first identified as a co-enzyme that imparts a hydrogen ion to ribonucleotide reductase, an essential enzyme for DNA synthesis in Escherichia coli. Human thioredoxin was cloned by Yodoi in 1989 as adult T-lymphocyte protein-derived factor (ATL-derived factor, or ADF). The active site of thioredoxin has the form -Cys-Gly-Pro-Cys-. It is well preserved in a wide range of organisms ranging from E. coli to mammals. It exists in an oxidized form in which a disulfide (S-S) bond is formed between two cysteines of an active site, and a reduced form in which a dithiol (-SH-SH) is formed at the same location. Thioredoxin is induced by various oxidative stresses including ultraviolet rays, radiation, oxidatns, viral infections, ischemia reperfusion or anticancer agents. Thioredoxin scavenges ROS (reactive oxygen species) by itself and in cooperation with peroxiredoxin. Thioredoxin plays a crucial roles in the redox regulation of transcription factors for the expression of various genes. Because the concentration of thioredoxin in human blood rises in the presence of different types of stress, measuring thioredoxin levels in the blood is considered to enable stress levels to be monitored. In addition, because mice with artificially increased thioredoxin levels exhibit resistance to stress and longer lifespans, it is expected that increasing the production of thioredoxin in the body or increasing its thioredoxin levels through ingestion or injection may enhance resistance to stress. |
Chemical Structure of Thioredoxin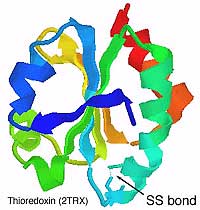
|